How Many Electrons Are in the Outer Shell of Aluminum
Aluminum contains 3 electrons in its outer shell thus it has 3 valence electrons. How many valence electrons are in a arsenic atom.
Chem4kids Com Aluminum Orbital And Bonding Info
Carbon Cstart message C end message en masse 14 component has 4 electrons in its outershell Carbon generally shares electrons to attain a full valence shell generating bonds with many various various other atoms.

. Thus the valence electrons aluminum have for bonding is 3. The general formula is that the nth shell can in principle hold up to 2 n2 electrons. 13 10 3.
Likewise how many electrons does cobalt have in its outer shell. Is the outer shell of carbon full. In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital.
The first 10 electrons are in the filled first and second shells. Aluminum aluminium is the element that is atomic number 13 on the periodic table. That means an atomic number of 8 oxygen has 8 protons and 8 electrons.
Aluminum has 14 positively charged protons and 14 negatively charged electrons giving it a net charge of zero. Shells hold up to two electrons in the first and eight electrons in the outer shells. It will are likely to lose three electrons and type a 3 ion.
The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on. The aluminum atomic number is 13 and has 11 protons in the nucleus. How many electrons are in the outer shell of aluminium.
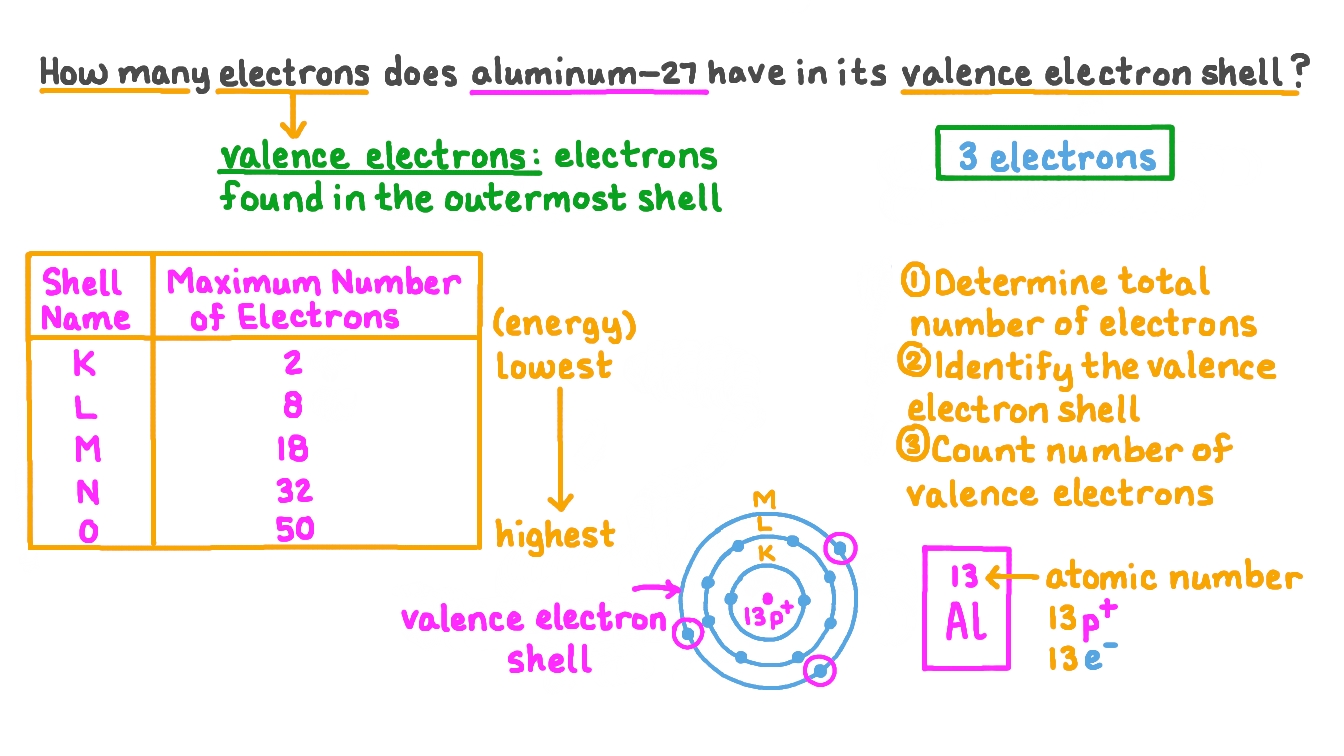
This leaves only three electrons in the third shell 3s23p1. Three electrons An aluminum atom will lose as much as three electrons when it types an ion creating the Al AL2 or Al3 cation. That means there are 13 electrons in a aluminum atom.
Thus the valence electrons aluminum have for bonding is 3. How many electrons does aluminum have in its outer shell. How many electrons can 2s hold.
They both readily form compounds in which they have six valence electrons rather than the usual eight predicted by the octet rule. Carbon Cstart text C end text as a group 14 element has four electrons in its outer shell. How many electrons are in the outer shell of electrons for uranium.
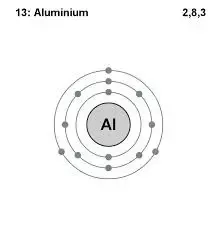
People also ask how many energy levels does aluminum have. Aluminum electron configuration That is the first shell of aluminum has two electrons the second shell has eight electrons and the 3rd shell has three electrons. Aluminum is a metal which have 3 valence electrons as there are 3 electrons in the outermost shell of the element.
Cobalt Co has an atomic number of 27 which means it has 27 electrons and protons. Three inner shells energy levels each containing six electrons leaving one empty level for the final valence electron. Howmany electrons fill the outer shell of carbon.
The electronic configuration of Antimony is 2 8 3. It has 13 electrons in its electron shell. Each shell can contain only a fixed number of electrons.
When we write the configuration well put all 13 electrons in orbitals around the nucleus of the Aluminium atom. How many electrons does aluminum have in its outer shell. Carbon typically shares electrons to achieve a complete valence shell forming bonds with multiple other atoms.
An arsenic atom has 33 electrons and 33 protons with five valence electrons those that can participate in forming chemical bonds with. Electrons is the number on the top right corner with the plus sign indicating that electrons are positive. Five valence electrons In the periodic table of the elements arsenic is No.
So there are only 3 valance electrons. A uranium atom has 92 protons and 92 electrons of which 6 are valence electrons. Uranium is a chemical element with the symbol U and atomic number 92.
What is 13 on the periodic table. In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom there are 13 electrons. What number of electrons does Aluminum achieve or lose.
So for the element of ALUMINUM you already know that the atomic number tells you the number of electrons. Aluminum is within the fifth column and due to this fact has 5 electrons in its outermost shell. The two elements that most commonly fail to complete an octet are boron and aluminum.
That means there are 13 electrons in a aluminum atom. The electron configuration for Aluminum is. It is a silvery-grey metal in the actinide series of the periodic table.
The electron configuration of the aluminum shows that there are two electrons in the K shell eight in the L shell and three in the M shellorbit. This leaves 12 electrons that are found outside of the nucleus. Looking at the picture you can see there are two electrons in shell one eight in shell two and three in shell three.
Aluminum is a metal which have 3 valence electrons as there are 3 electrons in the outermost shell of the element. All electrons in the outer shell are valence electrons Atoms tend to form ions or chemical bonds in order to end up with filled outer s and p subshells. Looking at the picture you can see there are two electrons in shell one eight in shell two and three in shell three.
Question Video Determining The Number Of Electrons In The Valence Electron Shell Of Aluminum 27 Nagwa

Aluminum Orbital Diagram Electron Configuration And Valence Electrons
Chem4kids Com Aluminum Orbital And Bonding Info

How Many Valence Electrons Does Aluminum Al Have

How Many Valence Electrons Does Aluminum Al Have
How Many Valence Electrons Are In Aluminium Quora

Chemistry Structure And Bonding Baamboozle

How Many Valence Electrons Does Aluminum Al Have

Electron Shell 013 Aluminium Aluminum Electron Shell Diagram Hd Png Download Transparent Png Image Pngitem
No comments for "How Many Electrons Are in the Outer Shell of Aluminum"
Post a Comment